Now, mask factories in European and American countries are facing some pressure: on the one…
Medical Mask Purchasing, Risk Control Series NO.1 – Validate the Certifications Before The Payment, Or You Might Loss the Money and Get Nothing!
Under the outbreak of COVID-19, masks became a hot commodity in 2020, but many companies(supplier and importer)were confused by various mask certifications. Masks have access conditions in many countries. In order to ensure that their masks can be exported smoothly, many manufacturers have to pay a large amount of money and time to ask the intermediary company for relevant certification, but things are not going as they wished.

Guangzhou Baiyun Airport Customs, which is affiliated to Guangzhou Customs, seized a batch of 100,000 surgical masks declared as “non-medical disposable protective masks”, and the manufacturing enterprises did not obtain the registration certificate of medical device products in China.
This situation brings a potential risk, i.e. if the masks you purchased can not pass the validation of the customs, you will lose your money and get nothing, so it is rather important to distinguish the authenticity of the certificate before you made the payment and confirm the order with any supplier.
But how?
In this article we will share a proper way to identify the mask suppliers you are dealing business with, each country has their own organization for medical mask trading and manufacturing qualifications with a specific registration number, we have gathered 5 countries or areas which are main medical face masks manufacturing and exporting areas.
01 China
Medical-Surgical masks belong to class II medical devices in China, which are registered and managed by provincial drug regulatory authorities. Medical devices can be checked through medical devices’ access numbers.
In order to read the information better we recommend installing a chrome plugin called “lingocloud”, this will help you translate Chinese to English with one simple click.
Do not use the English version of the website, the inquiry function is not available in the English version.
Then, open the official website, and click the lingocloud button to activate the translation.
https://www.nmpa.gov.cn/
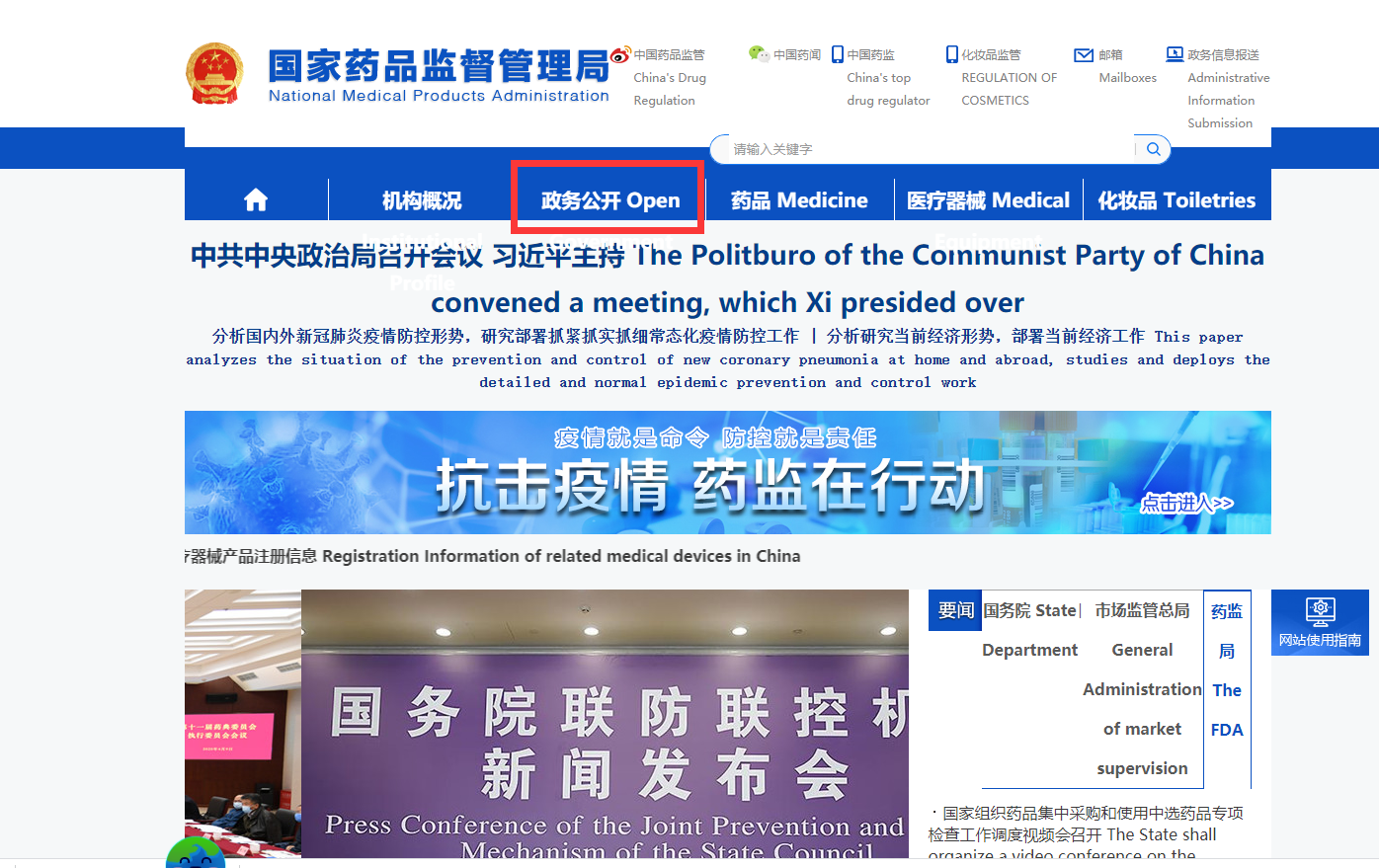 Navigate to the [政务公开Open Government] menu and click to the last submenu called [数据查询Data Inquiry]
Navigate to the [政务公开Open Government] menu and click to the last submenu called [数据查询Data Inquiry]
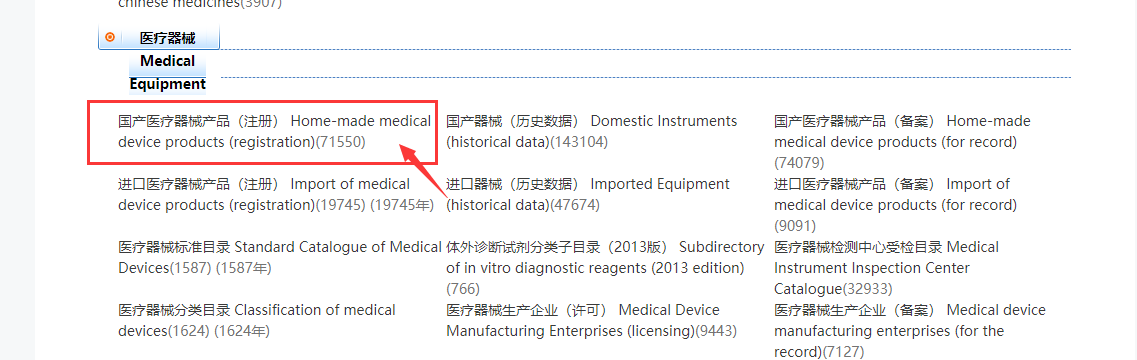
Click Lingocloud Plugin again to activate the translation, navigate to the Medical Equipment segment or you can Ctrl+F to search the number [71550], and click the link marked below:
You enter to the inquiry form page, then remember to active the Lingocloud plugin again, you get:
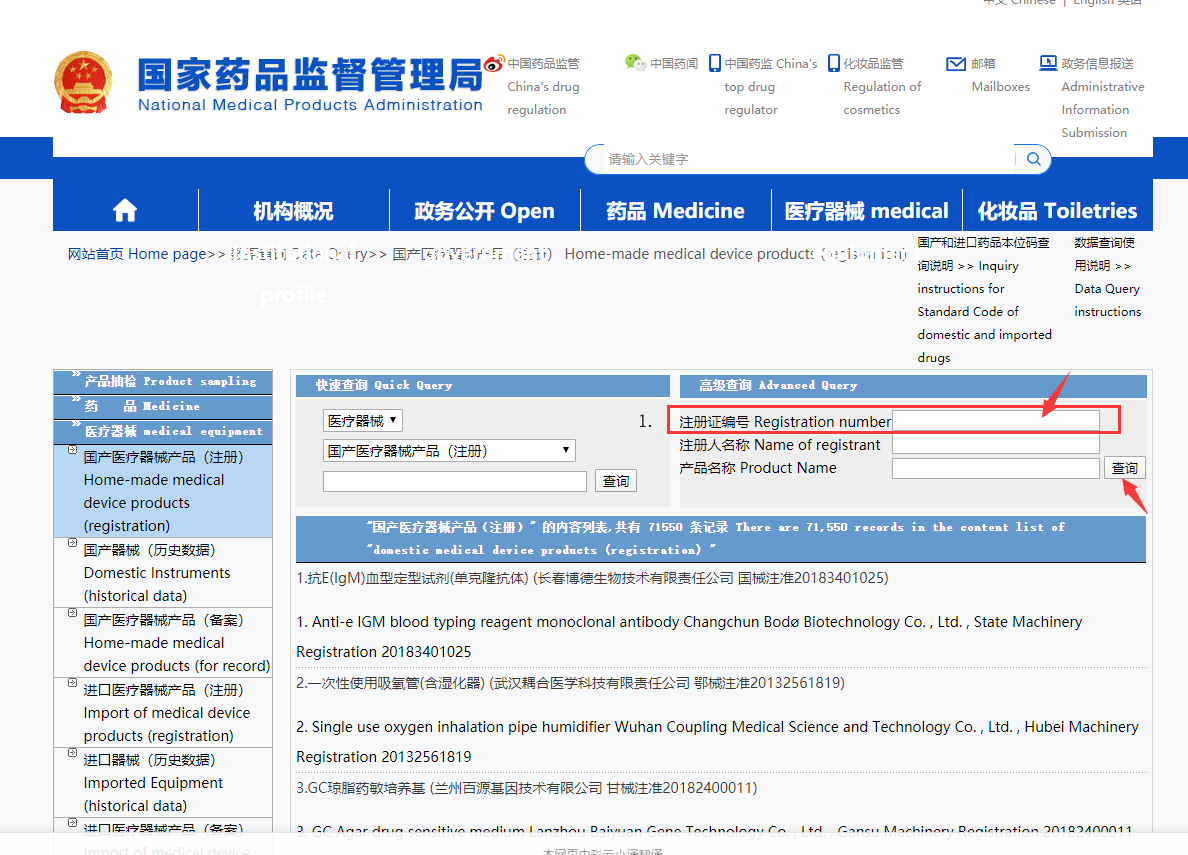
Enter the [Registration number] your supplier sent you, or you can find on the package.
A registration number consists of two parts, the Chinese part + 11 digits number
鄂械注准20172642430

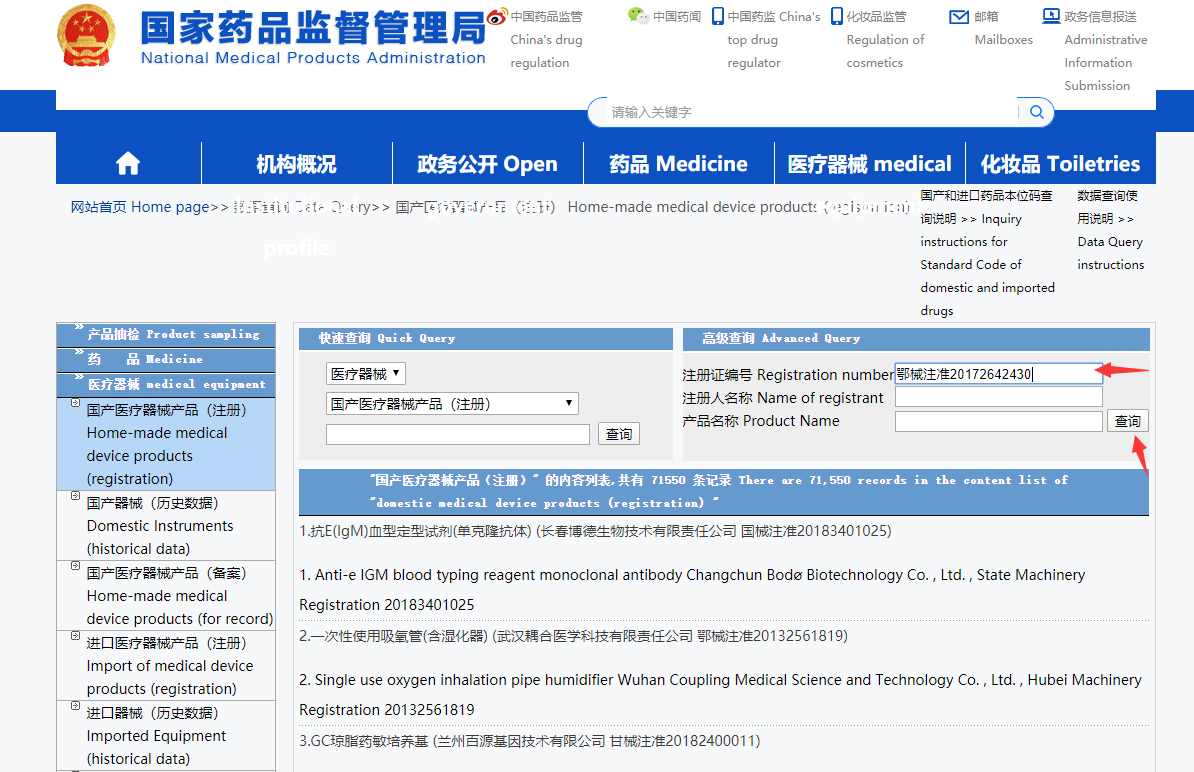
Put the registration number in the input area, and click the [查询] button, you can get the result.
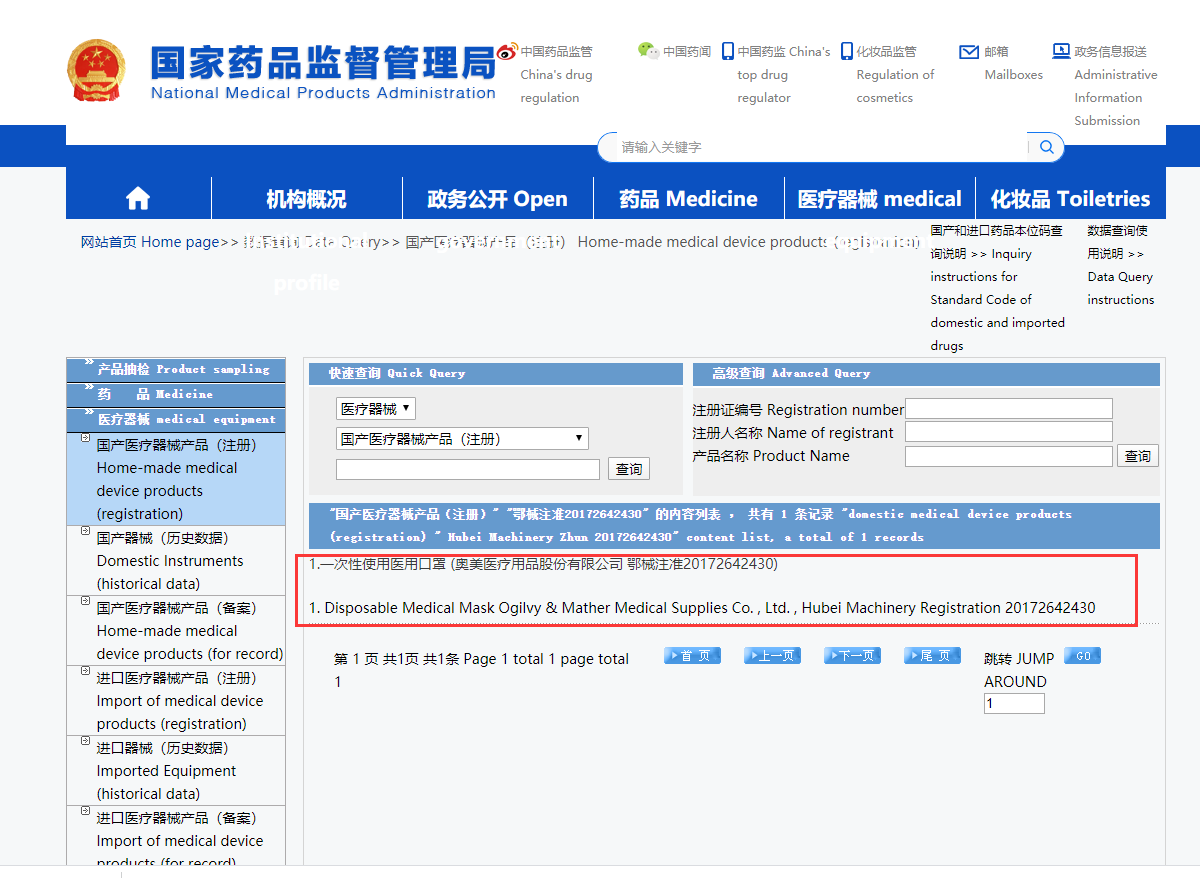
If you can get the result, then this registration number is a real one.
02 The United States
Surgical masks are medical devices in the United States and are regulated by the Food and Drug Administration (FDA). Recently, the FDA personally dispelled rumors and stated on the official website that it will not issue certifications to any enterprise.
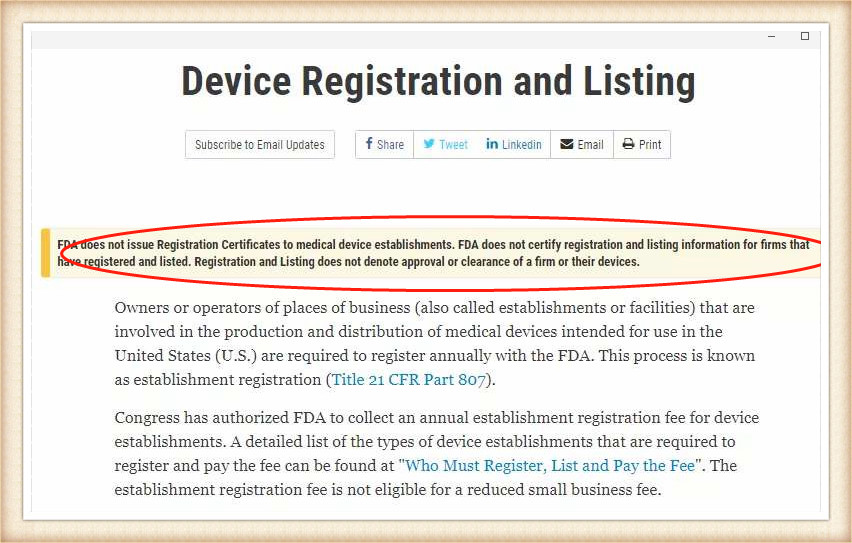
Mask products that have been approved by the FDA of the United States so far can inquire about the registration certificate number through their official website.
We recommend you to check the registration effectiveness via the FDA website:
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRL/rl.cfm
Open the above website, input the Registration Number or Owner Number on your certificate into the box as below.
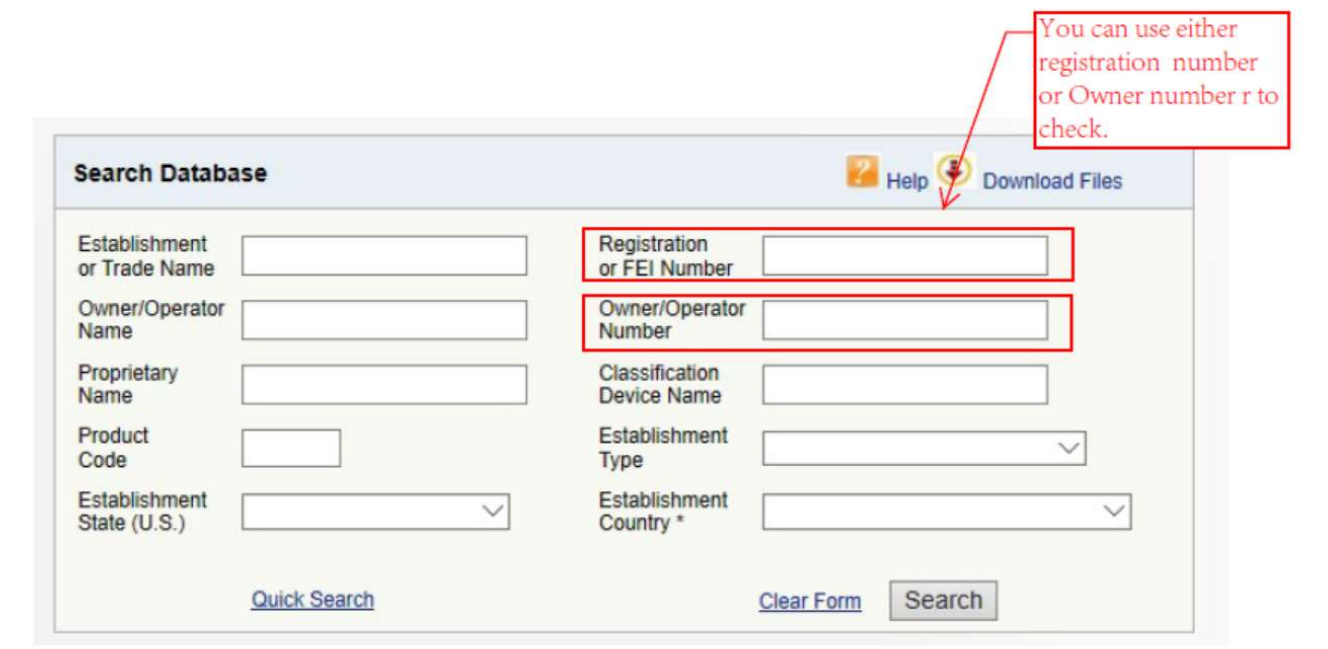
The search result will show you the facility information, as well as the device listed.
Important notice:Some manufacturers will claim that they have the FDA certifications by just providing you a so-called FDA certificate with an Owner/Operator Number but do not has registration numbers from FDA actually.
Example:
Below is the FDA certificate file, you might get a similar one from your supplier.

In the image above you can the there is an Owner/Operator Number:10063920,
From the FDA site you can get the result:
Https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRL/rl.cfm
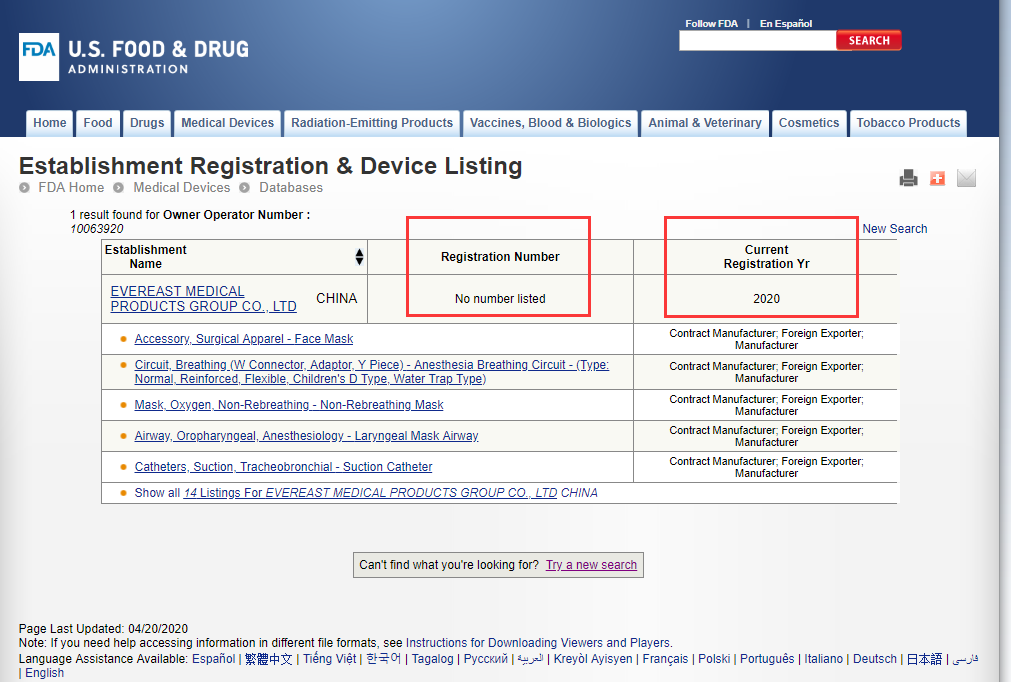
No number listed on the Registration Number section!
While the truth is: the results of the inquiries of companies that are really authorized by the FDA do have a registration number.
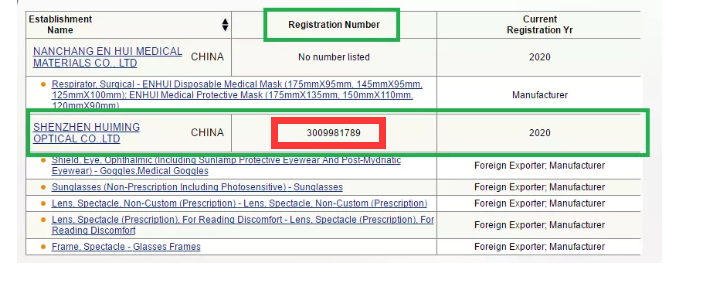
You can both get the factory information on the FDA’s official website, why some have registration numbers and some do not, what makes the difference?
What exactly does that Owner/Operator Number mean?
The answer is quite simple:
The Owner/Operator Number is equivalent to an application number.
Any company can apply for registration by submitting company and product information to the FDA.
Once submitted, the applicant automatically generates a number, which is the Owner/Operator Number.
But whether or not the FDA finally validate your application and gets you to register successfully and eventually get a registration number, it’s not for sure.
In other words, the so-called “FDA certificate” provided to you by the factory was actually an indication to you that they had submitted an application for registration to the FDA and the FDA had accepted it, that’s it!
If the products(medical masks)purchased from these suppliers by American customers are now airlifted to a certain customs in the United States, they will still not be cleared in the United States.
And according to the latest FDA policy, Chinese standard masks are currently approved subject to certain conditions.
The authorized enterprise link is https://www.fda.gov/media/136663/download
03 EU
The CE certifications on the market are varied and almost fake. An enterprise applying for certification can ask two questions from the certification authority:
01 Is your company an NB institution? Can the institution number be queried?
02 Can the CE certificate issued be checked on the official website?
 Common CE certification pseudo-certificates, pictures from the network
Common CE certification pseudo-certificates, pictures from the network
The European Union has announced a series of institutions authorized by the European Union for unified supervision and certification qualifications, also called NB institutions, and granted each institution a unique four-digit code, that is, the announcement number. The application and issuance of CE certificates are issued by the corresponding regulations and directives authorized by the announcement number agency.
The link is as follows.
https://ec.europa.eu/growth/tools-databases/nando/index.cfm?fuseaction=notifiedbody.
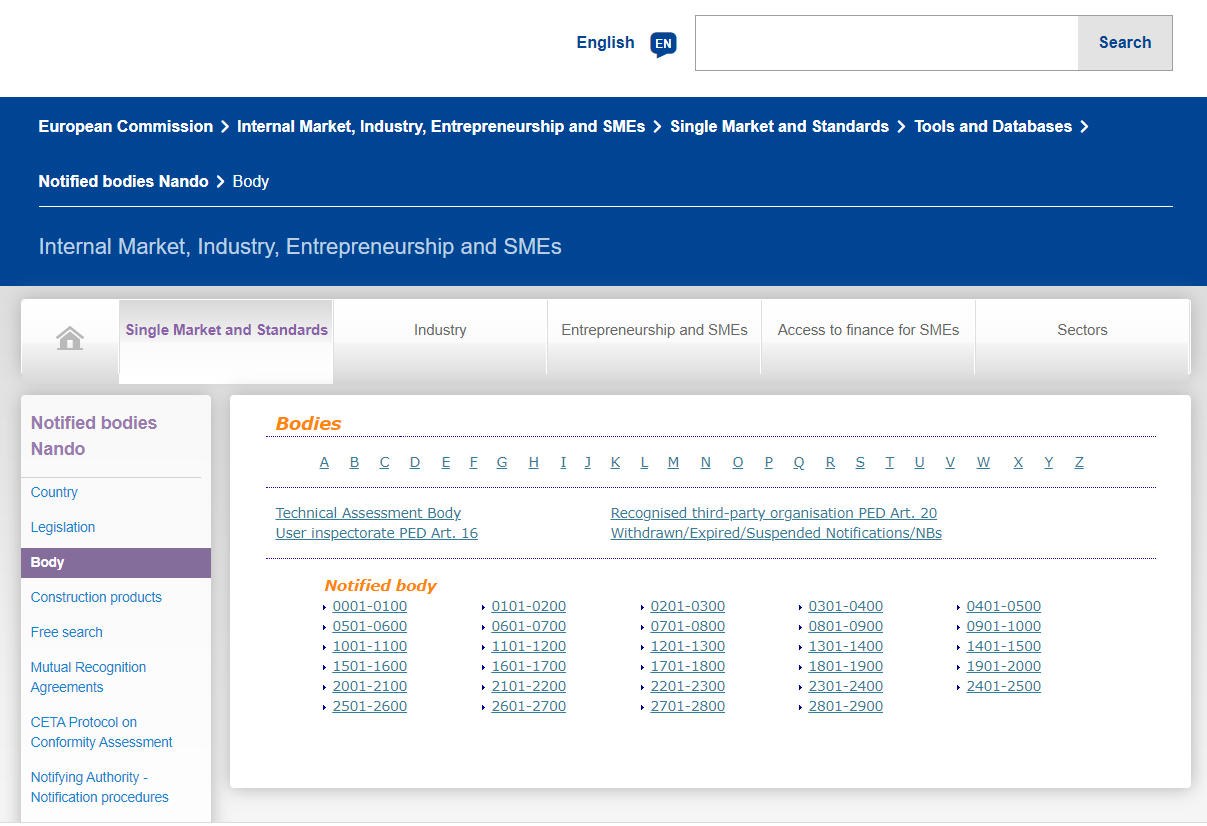
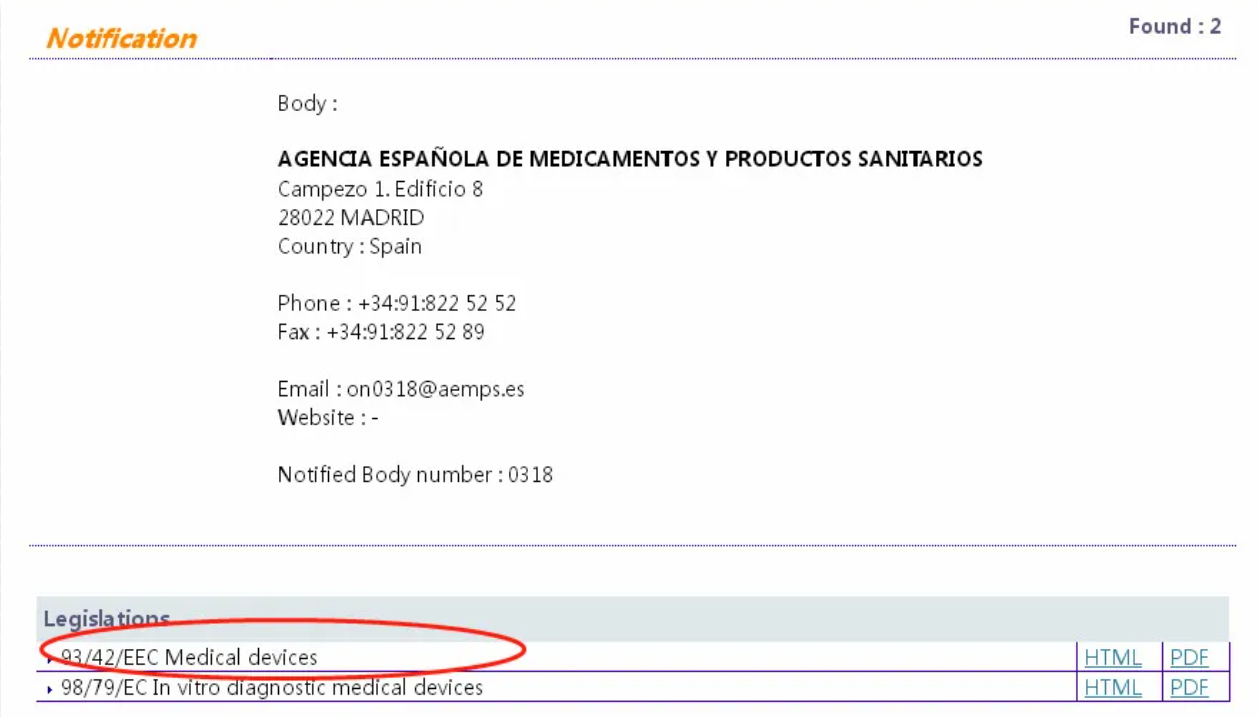
Corresponding to the obtained NB authorization number, click the corresponding code and enter to query the institution’s authorized directives. The certificate issued by the instruction within the scope of authorization is valid. Current EU directives related to masks are Medical Devices Directive 93/42 / EEC (MMD), new regulations on medical devices (EU) 2017/745 (MDR), Personal Protective Equipment (PPE) regulations (EU) 2016/425.
04 Korea
Medical surgical masks are class II medical device products in Korea. MFDS (Ministry of Food and Drug Safety of Korea) implements pre-market approval for Class II devices. South Korea stipulates that importers of such commodities should ensure that the imported products comply with the requirements of the quality management system and obtain permission from the MFDS authorized agency. MFDS only issues certificates to domestic companies. As Korea has a heavier responsibility, you don’t need to worry too much about the authenticity of the certificate.
05 Australia
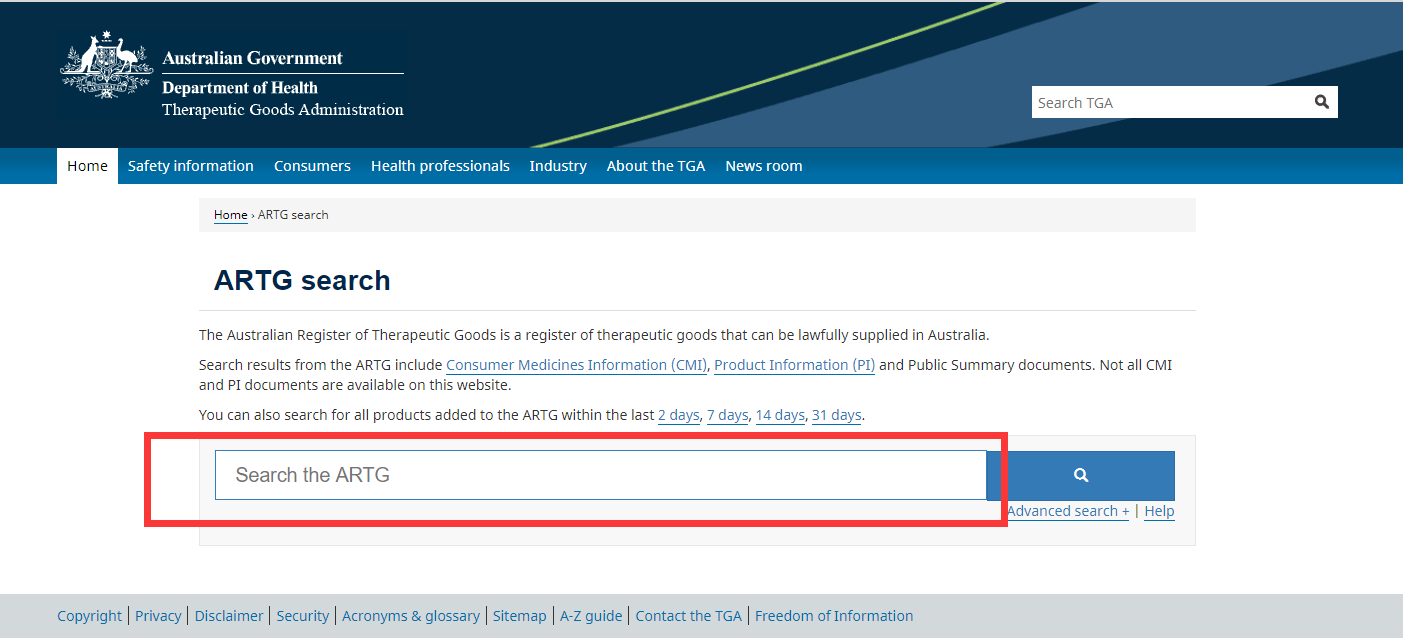
Mask products in Australia need to be registered by the Australian Government Health Products Authority TGA. TGA is the supervision agency for therapeutic products in Australia (including drugs, medical devices, genetic technology and blood products). According to Australian Therapeutic Goods (Medical Devices) Regulations 2002, Australia’s classification of medical devices is almost the same as that of the European Union. If it has obtained a CE certificate issued by the Notified Body of the European Union, it can be recognized by TGA and can be used as important registration information to meet Australian safety regulations. After the TGA is approved, an ATRG registration number will be generated. The query method is as follows.
First, enter the official website.
https://www.tga.gov.au/
 Select “Australian Register of Therapeutic Goods (ARTG)” in the box to start searching.
Select “Australian Register of Therapeutic Goods (ARTG)” in the box to start searching.
06 Conclusion:
Above methods to validate the certifications is suitable for importers who plan to purchase medical purpose face masks for health care workers, for general purpose daily non-medical masks(common 3 ply disposable face masks), there is no need for these certifications, these non-medical face masks are also proven to be effective to contribute to stop the spread of the COVID-19, and actually some of them are manufactured and tested to be qualified according to the medical standards, for example, in China, the disposable medical face mask’s standard is YY/T 0969, masks made implementing to this standard are effective for anti-virus purpose, so they are also a good alternative to medical face masks for common people, leave the medical/surgical face mask for doctors and nurses.
If you are interested in buying 3 ply disposable face masks, please check here.




This Post Has 0 Comments